October 25, 2021

US and Australia-based researchers have discovered a cheap new way to capture and convert CO2 emissions using liquid gallium.
The process can be done at room temperature and uses the metal to transform carbon dioxide into oxygen and a high-value solid carbon product that can later be used in batteries, for construction, or in aircraft manufacturing.
In a paper published in the journal Advanced Materials, the scientists explain that the newly discovered process dissolves captured CO2 gas into a solvent around nanoparticles of gallium, which exist in liquid state above 30°C.
The reactor also contains nano-sized solid silver rods that are the key to generating the triboelectrochemical reactions that take place once mechanical energy, for example, stirring or mixing, is introduced.
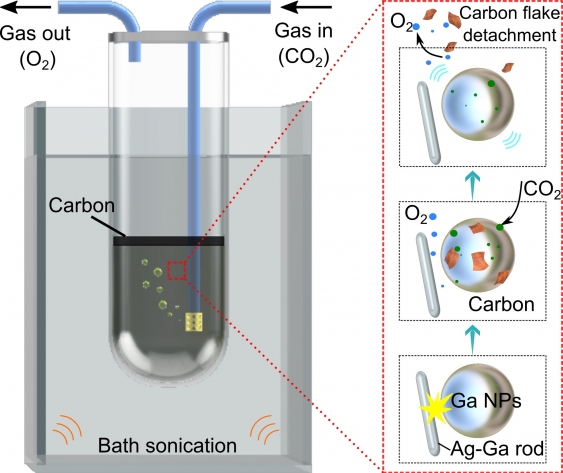
The reactions break the carbon dioxide into oxygen gas, as well as carbonaceous sheets which ‘float’ to the surface of the container due to differences in density and can therefore be easily extracted.
According to the research team, the process showed 92% efficiency in converting a tonne of CO2, using just 230kWh of energy. They estimate this equates to a cost of around $100 per tonne of CO2.
“We have already scaled this system up to two-and-a-half liters dimensions, which can deal with around 0.1 liter of CO2 per minute. And we’ve tested that running continuously for a whole month and the efficiency of the system did not degrade,” Junma Tang, first author of the study, said in a media statement.
“We see very strong industrial applications with regards to decarbonization. This technology offers an unprecedented process for capturing and converting CO2 at an exceptionally competitive cost.”
https://www.mining.com/liquid-metal-helps-convert-co2-into-useful-resources/

September 18, 2024

September 11, 2024

September 04, 2024